-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
- #Class 11
- #Medical
- #National Eligilibility Cum Entrance Test
- #Physics
- #Motion of System Of Particles and Rigid Body
A massless string connects two pulley of masses ' ' and '
' respectively as shown in the figure.
The heavier pulley is fixed and free to rotate about its central axis while the other is free to rotate as well as translate. Find the acceleration of the lower pulley if the system was released from the rest. [Given, ]
Not understanding sir
View Full Answer(2)- #Physics
- #BITSAT
- #Engineering
- #Class 11
- #Andhra Pradesh Engineering Agriculture and Medical Common Entrance Test
- #Laws of motion
Calculate the acceleration of block of the following diagram. Assume all surfaces are frictionless . Here m1 = 100kg and m2 = 50kg

0.33m/s2
0.66m/s2
1m/s2
1.32m/s2
1.32
View Full Answer(3)
Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

- #All India Institute of Medical Science MBBS
- #Medical
- #Biology
- #Jawaharlal Institute of Postgraduate Medical Education Research MBBS
- #Class 11
- #Cell Structure and Function
When cell has stalled DNA replication fork, which checkpoint should be predominantly activated?
G1/S
G2/M
M
Both G2 M and M
G2/M should be activated as the cell has stalled DNA replication fork.
View Full Answer(1)If 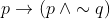 is false, then the truth values of p and q are respectively :
is false, then the truth values of p and q are respectively :
Option: 1 F, T
Option: 2 T, F
Option: 3 F, F
Option: 4 T, T
Relation Between Set Notation and Truth Table -
Sets can be used to identify basic logical structures of statements. Statements have two fundamental roles either it is true or false.
Let us understand with an example of two sets p{1,2} and q{2,3}.

Using this relation we get
-
Practise Session - 2 -
Q1. Write the truth table for the following statement pattern:
-
Correct Option (4)
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
- #Electromagnetic Induction and Alternating currents
- #Engineering
- #Physics
- #Joint Entrance Examination Main
In a fluorescent lamp choke ( a small transformer) 100 V of reverse voltage is produced when the choke current changes uniformly from 0.25 A to 0 in a duration of 0.025 ms. The self-inductance of the choke (in mH) is estimated to be _____.
Option: 1 10
Option: 2 20
Option: 3 30
Option: 4 40
At 300 K and 1 atm, 15 mL of a gaseous hydrocarbon requires 375 mL air containing 20% O2 by volume for complete combustion. After combustion, the gases occupy 330 mL. Assuming that the water formed is in liquid form and the volumes were measured at the same temperature and pressure, the formula of the hydrocarbon is :
Option: 1 C4H8
Option: 2 C4H10
Option: 3 C3H6
Option: 4 C3H8
Volume of N2 in air = 375 × 0.8 = 300 ml
Volume of O2 in air = 375 × 0.2 = 75 ml
15ml
0 0 15x -
After combustion total volume
330 = 300 + 15x
x = 2
Volume of O2 used
y = 12
So hydrocarbon is = C2H12
None of the options matches it therefore it is a BONUS.
----------------------------------------------------------------------
Alternatively Solution
15ml
0 0 15x -
Volume of O2 used
If further information (i.e., 330 ml) is neglected, option (C3H8 ) only satisfy the above equation.
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
An ideal gas undergoes a quasi static, reversible process in which its molar heat capacity C remains constant. If during this process the relation of pressure P and volume V is given by PVn=constant, then n is given by (Here CP and CV are molar specific heat at constant pressure and constant volume, respectively)
Option: 1
Option: 2
Option: 3
Option: 4
For a polytropic preocess
A point particle of mass m, moves along the uniformly rough track PQR as shown in the figure. The coefficient of friction, between the particle and the rough track equals µ. The particle is released, from rest, from the point P and it comes to rest at a point R. The energies, lost by the ball, over the parts, PQ and QR, of the track, are equal to each other, and no energy is lost when particle changes direction from PQ to QR. The values of the coefficient of friction µ and the distance x(=QR), are, respectively close to : 
Option: 1 0.2 and 6.5 m
Option: 3 0.2 and 3.5 m
Option: 4 0.29 and 6.5 m
Work done by friction at QR = μmgx
In triangle, sin 30° = 1/2 = 2/PQ
PQ = 4 m
Work done by friction at PQ = μmg × cos 30° × 4 = μmg × √3/2 × 4 = 2√3μmg
Since work done by friction on parts PQ and QR are equal,
μmgx = 2√3μmg
x = 2√3 ≅ 3.5 m
Applying work energy theorem from P to R
decrease in P.E.=P.E.= loss of energy due to friction in PQPQ and QR
where h=2(given)
Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

- #Some Basic Principles of Organic Chemistry
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
In the following structure, the double bonds are marked as I, II, III and IV  Geometrical isomerism is not possible at site (s) :
Geometrical isomerism is not possible at site (s) :
Option: 1 III
Option: 2 I
Option: 3 I and II
Option: 4 III and IV
Geometrical isomerism is not possible at Site I as two identical methyl groups are attached to the same carbon bearing the double bond.
Hence, the answer is Option (2)
View Full Answer(1)- #Engineering
- #Chemistry
- #Classification of Elements and Periodic table
- #Joint Entrance Examination Main
Which one of the following is an oxide ?
Option: 1 KO2
Option: 2 BaO2
Option: 3 SiO2
Option: 4 CsO2
SiO2 - oxide
KO???2? , CsO????2 - superoxides
BaO????2 - peroxide
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE



